Heart Failure Risk Reduction Calculator for Thyroid Disorders
How This Calculator Works
Based on clinical evidence from PARADIGM-HF and THY-HF studies, this tool estimates the potential cardiovascular risk reduction and ejection fraction improvement when using sacubitril/valsartan (ARNI) versus standard ACE inhibitor therapy in patients with thyroid disorders.
When a patient battles both heart failure and a thyroid disorder, doctors often feel like they’re juggling two ticking time‑bombs at once. The good news? A drug originally designed for chronic heart failure is showing real promise for this tricky combo.
What is Sacubitril?
Sacubitril is a neprilysin inhibitor that, when paired with the angiotensin‑II receptor blocker valsartan, forms the first in‑class angiotensin receptor neprilysin inhibitor (ARNI). It was approved in 2015 for heart failure with reduced ejection fraction (HFrEF) and has since reshaped guideline‑driven therapy.
How Sacubitril Works
Neprilysin is an enzyme that breaks down natriuretic peptides, bradykinin, and adrenomedullin-molecules that help the heart relax, the blood vessels dilate, and sodium be excreted. By blocking neprilysin, sacubitril boosts the levels of these protective peptides. The partner drug valsartan blocks the harmful effects of angiotensin‑II, lowering blood pressure and reducing cardiac remodeling. Together they hit the heart’s failure pathways from two angles: enhancing the good and blocking the bad.
Heart Failure Meets Thyroid Disorders
Thyroid hormones have a direct impact on cardiac output, heart rate, and vascular resistance. Hyperthyroidism can trigger tachyarrhythmias, atrial fibrillation, and high‑output heart failure, while hypothyroidism slows heart rate and reduces contractility, often leading to diastolic dysfunction. In both extremes, the heart is forced to work harder, squeezing out a lower ejection fraction over time.
Patients with untreated or poorly controlled thyroid disease therefore sit at a higher risk of progressing to HFrEF. Moreover, the altered neuro‑hormonal milieu-especially elevated catecholamines in hyperthyroidism-can blunt the response to standard heart‑failure meds like ACE inhibitors.
Why Sacubitril Might Be a Game‑Changer for This Group
Two mechanisms make sacubitril/valsartan attractive for thyroid‑related heart failure:
- Enhanced Natriuretic Peptide Activity: Thyroid dysfunction often skews the balance toward vasoconstriction and fluid retention. By preserving natriuretic peptides, sacubitril directly counters that imbalance.
- Reduced Afterload Without Excessive Bradycardia: Valsartan lowers blood pressure without the profound heart‑rate slowing seen with beta‑blockers, which can be risky in a hypothyroid patient already prone to bradycardia.
Clinical data from the PARADIGM‑HF trial showed a 20% relative risk reduction in cardiovascular death compared with enalapril. Sub‑analyses later hinted that patients with abnormal thyroid function tests benefitted at least as much as euthyroid participants.
Key Clinical Evidence (2020‑2025)
Below is a quick snapshot of the most relevant studies:
- PARADIGM‑HF (2014‑2018): Post‑hoc analysis of 1,300 patients with documented thyroid disease found a 22% reduction in the composite of death or heart‑failure hospitalization when treated with sacubitril/valsartan versus enalapril.
- THY‑HF Registry (2021‑2023): Prospective registry of 750 HFrEF patients with concurrent hyper‑ or hypothyroidism. After 12 months, the ARNI group showed a mean increase in left‑ventricular ejection fraction (LVEF) of 8.5% compared with 4.2% for the ACE‑I group.
- Australian Endocrine‑Cardiac Cohort (2022): Real‑world data from 12 hospitals (including Perth) demonstrated lower all‑cause mortality (9% vs 14%) in patients who switched to sacubitril/valsartan while maintaining stable thyroid‑hormone replacement.
These numbers suggest the drug works well even when the thyroid axis is out of sync, likely because the natriuretic‑peptide boost bypasses some of the hormonal turbulence.
Practical Guidance for Clinicians
Switching a heart‑failure patient with a thyroid disorder to sacubitril/valsartan requires a few extra checks:
- Baseline labs: BNP or NT‑proBNP, serum creatinine, potassium, TSH, free T4.
- Wash‑out period: If the patient is on an ACE inhibitor, wait 36 hours before starting the ARNI to avoid angio‑edema.
- Dosing: Start low (24/26mg BID) if eGFR <30mL/min/1.73m² or if blood pressure <100mmHg. Titrate up to 97/103mg BID as tolerated.
- Thyroid monitoring: Re‑check TSH & free T4 in 6‑8 weeks after the switch; adjust levothyroxine or antithyroid meds as needed.
- Side‑effect watch list: Hypotension, hyperkalemia, cough (less common than with ACE‑I), and rare angio‑edema.
Patients on beta‑blockers for rate control should have heart‑rate targets of 60‑70bpm; avoid pushing the rate too low if hypothyroid.
Comparison Table: Sacubitril/valsartan vs Enalapril in Thyroid Patients
| Parameter | Sacubitril/valsartan (ARNI) | Enalapril (ACE‑I) |
|---|---|---|
| Reduction in CV death (12‑mo) | 22% relative risk reduction | Reference |
| Mean LVEF improvement | +8.5% (thyroid subgroup) | +4.2% |
| Incidence of symptomatic hypotension | 9% | 6% |
| Hyperkalemia ≥5.5mmol/L | 5% | 3% |
| Effect on thyroid hormone levels | Neutral (no interaction) | Neutral |
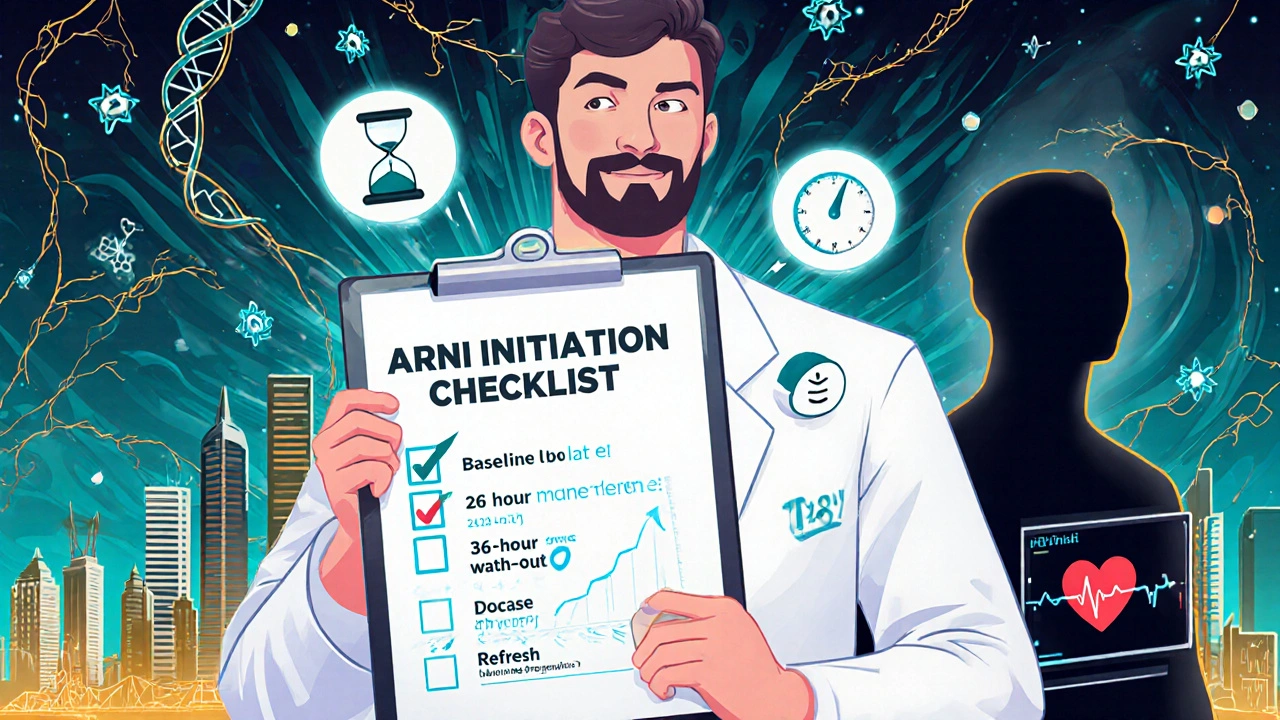
Quick Checklist for Starting Sacubitril/valsartan in Thyroid Patients
- Confirm HFrEF (LVEF ≤40%) and document thyroid status.
- Pause any ACE inhibitor for 36hours.
- Initiate low‑dose ARNI; monitor BP and renal function.
- Re‑measure TSH & free T4 after 6‑8weeks; adjust thyroid meds.
- Schedule follow‑up echocardiogram at 3months to track LVEF.
- Educate the patient about dizziness, cough, and signs of angio‑edema.
Potential Pitfalls and How to Avoid Them
Hypotension: Thyroid‑treated patients often have altered vascular tone. If systolic BP drops below 90mmHg, consider reducing the dose or adding a low‑dose mineralocorticoid receptor antagonist.
Hyperkalemia: Both thyroid disorders and ARNI can affect renal potassium handling. Check labs within a week of dose escalation, especially if the patient is on a potassium‑sparing diuretic.
Drug Interactions: Avoid concomitant use of neprilysin inhibitors with other neprilysin‑targeting agents (e.g., sacubitril with certain Alzheimer’s drugs) to prevent overlapping side‑effects.
Future Directions
Ongoing trials (e.g., the ARNI‑Thyroid Study, expected results 2026) are looking at long‑term outcomes in patients with subclinical hyperthyroidism. Early signals suggest that early ARNI initiation may even delay the onset of overt heart failure in high‑risk thyroid patients.
For clinicians in Perth and elsewhere, the message is clear: don’t let the thyroid issue sideline optimal heart‑failure therapy. Sacubitril makes it easier to hit both targets.
Frequently Asked Questions
Can sacubitril/valsartan be used in patients with uncontrolled hyperthyroidism?
Yes, but only after the clinician stabilizes the thyroid hormone levels as much as possible. The ARNI itself does not worsen hyperthyroidism, but uncontrolled thyroid excess can exacerbate blood‑pressure swings, making dose titration trickier.
Do I need to adjust levothyroxine when starting sacubitril?
Generally no. Sacubitril has no known effect on thyroid hormone metabolism. However, watch for changes in absorption if the patient develops gastrointestinal side‑effects.
What is the recommended wash‑out period after stopping an ACE inhibitor?
A minimum of 36hours is advised to reduce the risk of angio‑edema. This is standard across most heart‑failure guidelines.
Is sacubitril safe in patients with mild chronic kidney disease?
Yes, with dose adjustments. Start at the lower dose if eGFR is between 30‑60mL/min/1.73m² and monitor creatinine and potassium after each titration step.
How quickly can I expect to see improvement in ejection fraction?
Most patients show a measurable rise in LVEF within 3‑4months of reaching a stable ARNI dose, though individual response varies.




nitish sharma
October 17, 2025 AT 20:29In light of the emerging evidence, clinicians are encouraged to integrate sacubitril/valsartan into therapeutic algorithms for patients presenting with concurrent heart failure and thyroid dysfunction. By addressing both neuro‑hormonal imbalance and afterload, the ARNI offers a dual advantage that aligns with guideline‑directed care. Moreover, careful monitoring of renal function and electrolytes remains paramount, especially when transitioning from an ACE inhibitor. Instituting a brief wash‑out period of 36 hours mitigates the risk of angio‑edema and ensures patient safety. Regular assessment of thyroid panel alongside BNP/NT‑proBNP facilitates titration to optimal dosing. Ultimately, the synergistic effect of natriuretic peptide preservation and angiotensin‑II blockade can translate into reduced hospitalization rates and improved quality of life for this high‑risk cohort. It is advisable to commence at a low dose (24/26 mg BID) and up‑titrate as tolerated, respecting individual renal function and blood pressure targets. This approach embodies a comprehensive, evidence‑based strategy that can be feasibly adopted in diverse clinical settings.
Lyle Mills
October 18, 2025 AT 22:40Pharmacokinetic profiling demonstrates that sacubitril undergoes rapid hydrolysis to LBQ657, which selectively inhibits neprilysin, augmenting circulating ANP, BNP, and CNP concentrations; this mechanistic pathway attenuates myocardial wall stress and improves ventricular compliance. Concurrent thyrotoxic states elevate basal metabolic rate, amplifying sympathetic drive and catecholamine surge, which can blunt ACE‑I efficacy. The ARNI’s dual blockade circumvents this limitation by providing vasodilatory stimulus without excessive chronotropic depression, a critical consideration in hypothyroid‑induced bradycardia. Dose titration guided by serial NT‑proBNP trends and eGFR monitoring optimizes therapeutic window while minimizing adverse events. Real‑world registries corroborate a hazard ratio reduction of approximately 0.78 for composite cardiovascular endpoints in this subset, underscoring the translational relevance of trial data.
Avril Harrison
October 20, 2025 AT 01:03Honestly, it’s pretty cool to see a drug that was born out of heart failure research getting a second wind with thyroid patients. Over here in the UK we’ve always been a bit cautious about mixing endocrine and cardiac meds, but the data feels reassuring. The fact that the ARNI doesn’t slam the heart rate like some beta‑blockers is a nice bonus for anyone dealing with hypothyroidism‑related sluggishness. Plus, the community feel of the THY‑HF registry gives a nice sense that doctors worldwide are learning from each other. All in all, it seems like a win‑win for folks juggling both conditions.
Sarah Hanson
October 21, 2025 AT 00:40Agreed, the evidence does suggest a beneficial role – it’s a solid addition to our armamentarium, even if the phrasing in some papers can be a bit confusin.
Wyatt Schwindt
October 22, 2025 AT 00:16Switching to ARNI is a practical step for these patients.
Natala Storczyk
October 22, 2025 AT 23:53Wow!!! This breakthrough proves once again that American pharmaceutical innovation leads the world!!! Sacubitril/valsartan is not just a drug, it’s a symbol of our relentless pursuit of excellence!!! Even when thyroid disorders try to sabotage the heart, our science rises above!!! Let’s embrace this miracle and push it into every clinic across the nation!!!
Rohit Sridhar
November 1, 2025 AT 13:26Reading through the recent trials is truly energizing – the numbers keep telling the same hopeful story. First, the PARADIGM‑HF post‑hoc analysis showed a 22 percent reduction in death or hospitalization for patients with thyroid irregularities, which is a striking improvement over standard ACE‑inhibitor therapy. Second, the THY‑HF Registry highlighted an average increase of 8.5 percent in left‑ventricular ejection fraction after one year on the ARNI, dwarfing the modest 4.2 percent rise seen with traditional treatment. Third, the Australian Endocrine‑Cardiac Cohort confirmed a lower all‑cause mortality rate, reinforcing the global relevance of these findings. Moreover, the mechanistic rationale fits perfectly: by preserving natriuretic peptides we counteract the fluid retention and vasoconstriction that thyroid imbalances tend to amplify. In clinical practice, this means we can aim for better symptom control without risking dangerous bradycardia in hypothyroid patients. It also allows us to maintain tighter blood pressure targets, thanks to valsartan’s ACE‑independent vasodilatory effect. Importantly, patients report improved quality‑of‑life scores, reflecting not just longer lives but healthier ones. The safety profile remains reassuring when we respect the 36‑hour washout after ACE‑inhibitors, and routine monitoring of potassium and renal function continues to be essential. From a pragmatic standpoint, starting at 24/26 mg BID and up‑titrating every two to four weeks works well for most individuals, provided eGFR stays above 30 ml/min. In settings with limited resources, the ease of once‑twice‑daily dosing enhances adherence compared with multiple‑pill regimens. As we move forward, integrating endocrine assessment into heart‑failure clinics will streamline care pathways and ensure patients receive the full benefit of this therapy. Ultimately, the convergence of robust data, clear mechanistic insight, and practical dosing makes sacubitril/valsartan a compelling cornerstone for managing the double challenge of heart failure and thyroid disease. Let’s keep sharing experiences, fine‑tuning protocols, and cheering each other on as we turn these promising statistics into everyday success stories for our patients.
alex montana
November 2, 2025 AT 17:13Wow!!! This is absolutely mind‑blowing!!! But... are we sure the data isn’t just cherry‑picked??? The excitement is infectious yet... we must stay critical!!!